DFG Research Unit FOR 2685 | C2
Soft tissue preservation in amber – a comparative study on the taphonomy and limits of fossilization of resin embedded arthropods
Abstract
Amber is one of the most important sources of fossil insects and gives insight into Mesozoic and Cenozoic terrestrial ecosystems (Fig 1). The fossilized resin preserves trapped organisms with a unique cellular and ultra-structural fidelity, but the processes leading to this exceptional conservation are still not understood. The characteristic of Indian amber to be completely soluble in certain solvents opens up new possibilities on the examination of amber preservation. Integration of extant, and museum samples, as well as copal alongside amber collections around the world, will lead to the most extensive study on amber inclusion taphonomy so far.

Figure 1 | Ant (Formicidae) and mite (Acari) in Baltic amber (~ 50 Ma).
Many reports note the excellent conservation of musular, digestive, respiratory, nervous, and sensory tissues in amber [1-4]. Large differences in organic compound preservation between amber deposits [5-6] imply diverse taphonomic pathways of the fossil organisms. A known phenomenon is the preservation as an outer cast, with specimens being hollow inside [7].This type often occurs in Baltic amber, but for the Lebanese and Indian amber real body fossils expressing cuticle preservation are evidenced [8-9]. These differences in preservation may refer to crucial factors such as the depositional environment, the resin compounds, or the geologic age.
Several inclusions of Indian Cambay amber have been extracted with organic solvents (Fig. 2) [11]. Different types of internal soft tissues are preserved with lifelike fidelity, allowing detailed examinations not only with SEM but also with TEM, Raman spectroscopy, histology or even histochemical methods (Fig. 3). A non-destructive screening of promising specimens has been carried out at the DESY in Hamburg and will be continued throughout the project in combination with Micro-CT scans. Raman spectroscopy of an Anolis sp. limb in Dominican amber revealed large modifications in the organic parts of the bone and verified this method as a viable tool with regard to the project.

Figure 2 | Midge extracted from Indian amber (~ 53 Ma).
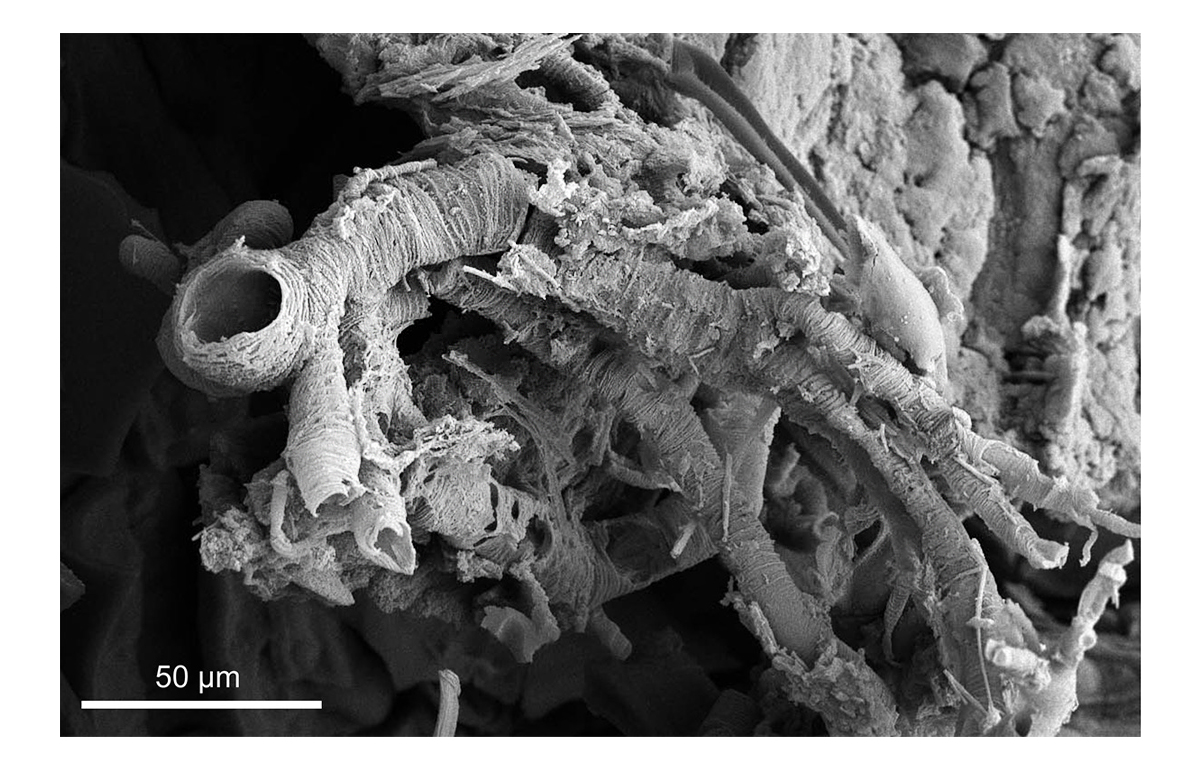
Figure 3 | SEM image of the tracheal system of an extracted beetle from Indian amber (~ 53 Ma).
- Examination of the different processes that affect the diverse preservation of resin embedded arthropods. Specimens will comprise extant material, museum samples, and amber fossils as well as copal inclusions.
- Identifying the limits of amber preservation with a focus on cellular components and chemical investigations of different tissues and organ systems.