DFG Research Unit FOR 2685 | A2
Organic Phase (Extracellular Matrix, Osteocyte, Blood Vessel) Preservation in Dinosaur Bone: Chemical Composition and Hypotheses of Preservation
Abstract
The aim of the project is two-fold: first, to test the hypothesis that the organic phase (OP) represents altered extracellular matrix (ECM), osteocytes, and blood vessels (vs. fossilized biofilms of degrading bacteria). The second aim is to understand the processes leading to the preservation of the OP (and its degradation products) vs. bacterial biofilms and to test hypotheses of which conditions are conducive to preservation of organic compounds in fossil bone in general.
In addition to their shape, fossil bones also preserve their histological structure, giving rise to the field of vertebrate paleohistology. Bone is a composite material that consists of 70% by volume of the bone mineral hydroxyapatite (inorganic phase; IP) and 30% of collagen and other minor organic constituents (organic phase; OP). During bone formation, both blood vessels and bone-forming cells become incorporated into the bone matrix, adding to the OP. During the fossilization of bone, the collagen part and most or all of the original hydroxyapatite is replaced by fluorapatite (FAp). Thus, fossil bone is highly mineralized, largely consisting of FAp and diagenetic mineral fillings in the spaces of former blood vessels (vascular spaces).
While it has been known since the 1960s that OP and bone proteins can be detected in fossil dinosaur bone, it has only recently been recognized by Mary Schweitzer that osteocyte remains and blood vessels can be liberated from well- mineralized fossil dinosaur bone by careful etching with weak organic acids (Fig. 1). This discovery led to a controversy over the nature of the pliable organic remains as OP vs. biofilms produced by bone-degrading bacteria.

Figure 1 | Isolated osteocytes and flexible blood vessels extracted from a 150 MA thigh bone of a sauropod dinosaur from the Morrison Formation, Wyoming, USA. Note the spiral banding in the vessels (right), suggestive of collagen preservation. Original image by M.C. Koschowitz.
Applicant PMS has done extensive work on fossil bone, from the histological level down to the nanometer scale (Fig. 2). Our group recently has attempted to reproduce the results of Schweitzer, but with a much greater temporal and environmental sample range. In a preliminary study, we were able to isolate osteocyte remains as old as the Early Permian (290 MA) and blood vessel remains from the Late Jurassic (150 MA) (Fig. 1). Remarkably, the fossil bone samples that preserved bone tissue are not from conservation deposits, but from normal fluvial and marine facies (Fig. 3). We currently lack particular predictive factors for OP preservation, and the question remains as to the material nature of the OP found in fossil bone.
The lab of CEM is very experienced in analytical and bioanalytical chemistry, molecular biology (Western blot and antibodies) as well as synthetic organic chemistry. In collaboration with ME (PI in A3), we analyzed 66 MA dinosaur eggshell and identified two tetrapyrrolic pigments. CEM currently works on hematin detection from bone (Fig. 4).
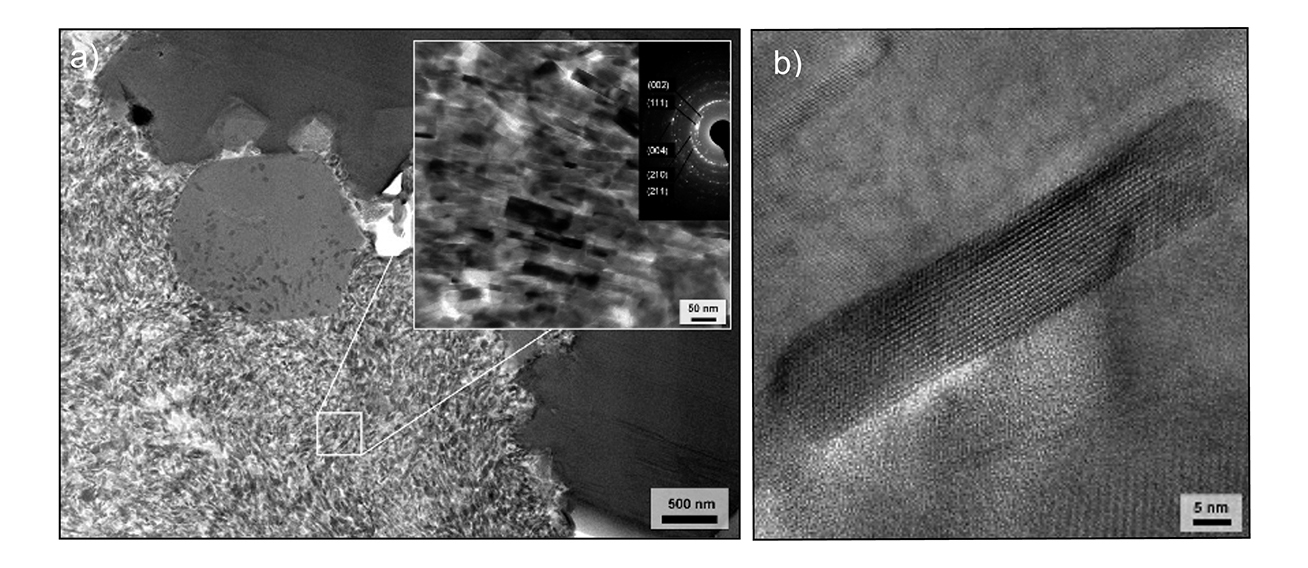
Figure 2 | a) TEM image of an Apatosaurus sp. bone sample (Jurassic, USA) showing submicrometer structure consisting of preserved bone crystallites around a vascular canal. The inset shows the nano-size FAp crystals of the bone and the associated diffraction pattern. b) Single fossil bone apatite crystallite from a Jurassic sauropod dinosaur long bone imaged using TEM. The parallel lines are the crystal lattice of the FAp at the atomic scale! Modified from [1].
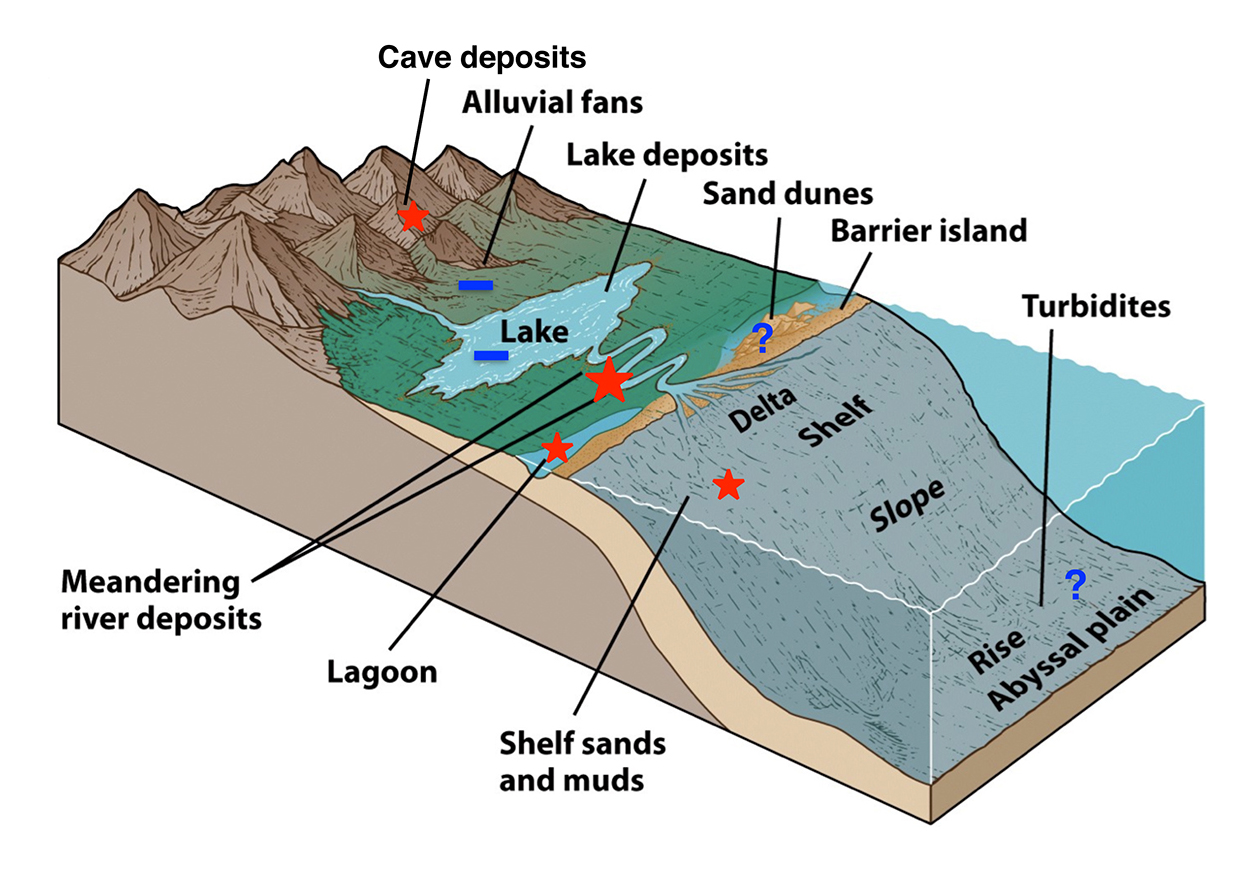
Figure 3 | Association of sedimentary facies and OP preservation based on the preliminary work by the applicants. Note that lakes and alluvial fans do not appear to be conducive to OP preservation, but fluvial sediments are best.
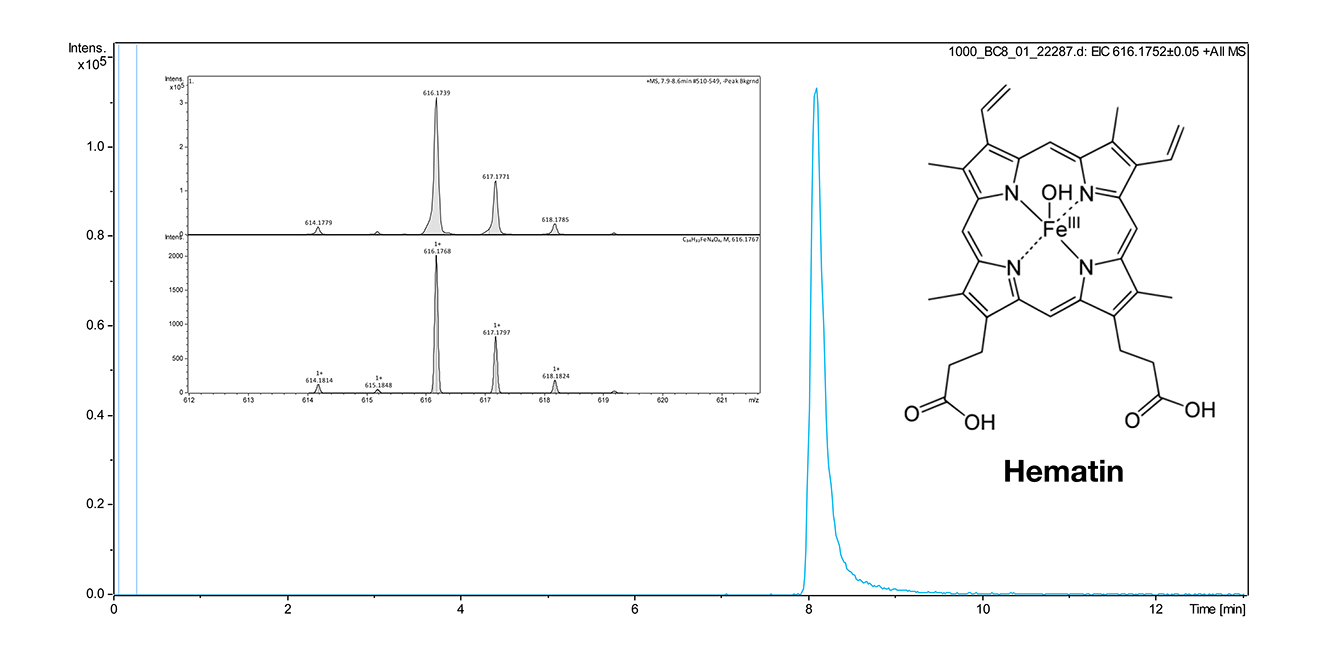
Figure 4 | Structure and method for hematin detection by LCMS. An HPLC Dionex Ultimate 3000 (Thermo Scientific) with integrated wavelength detector coupled with 1.0 a mass spectrometer micrOTOF-Q with ESI source (Bruker) was used. Column: EC50/2 Nucleodur C18 Gravity 3 μm (Macherey & Nagel). Gradient 40% aq. MeOH containing 2 mM ammonium acetate to 1000 % MeOH containing 2 mM ammonium acetate within 9 min. The extracted ion chromatogram (EIC) from the molecular peak at 616.174 was used for quantification. The limit of detection was ca. 100 nM and will be improved in subsequent experiments, also by using a projected triple quadrupol MS instrument. Bones will be crushed by using a swing mill (at 30 Hz for 1 min). Extraction of hematin and degradation products will be performed with a solution of 0.5%-1% NH3 in water followed by lyophilization.
1st funding period:
- H 1: Microscopically visible soft parts are altered ECM, osteocytes, and blood vessels [2,3,4,5]).
- H 2: Microscopically visible soft parts are altered microbial products of decay [6].
- H 3: Original collagen banding is preserved in fossil bone [4,7].
- H 4: Organic compounds in chemical dinosaur bone extracts are derived from original OP [2,5].
- H 5: Original organic compounds or their degradation products can be detected in situ [2,3,8].
- H 6: Organic preservation is linked to ferruginous mineralizations [2,3,8].
- H 7: Microbial exoenzymes such as collagenase are involved in the preservation of the bone OP.
- H 8: OP in bone is best preserved in fluvial environments [9].
- H 9: OP preservation in bone is independent of geologic age [9].
2nd funding period:
Analysis of the preserved compounds from an evolutionary and paleoecological perspective [10].