DFG Research Unit FOR 2685 | A3
The organic phase in dinosaur eggshell: analysis, models of preservation, and implications for the evolution of bird reproduction
Abstract
The general goal of the project is to understand the material nature of dinosaur and Mesozoic bird eggshell. The focus will be on the organic phase (which remains largely unstudied as opposed to the mineral phase), exploring biomolecular preservation in eggshell. Eggshell offers an ideal matrix for such preservation, corroborated by our preliminary work. We intend not only to detect, locate, and identify preserved pigments and degradation products thereof, but there is potential for the preservation of the protein compounds that build up the squamatic scaffold in eggshell. Our research is set against the background of the evolution of avian reproductive traits for which a refined understanding of the fossil egg record is crucial.
It has long been recognized that fossil eggs and eggshells (Fig. 1) preserve their histological structure, giving rise to the field of eggshell paleohistology. Eggshell is a biocomposite material just like bone, but it is mineralized to a much higher degree. Eggshell represents a more promising matrix for the preservation of endogenous biomolecules than bone because it is less affected by dissolution-remineralization processes during fossilization. This stable, crystalline matrix contains minor amounts of organic constituents, the organic phase (OP), which plays a role in embryo development, strengthening of the eggshell, and egg coloration. Composite systems of biominerals and organic constituents are known to form a local chemical environment that allows the almost unmodified preservation of its organic constituents over geological time.
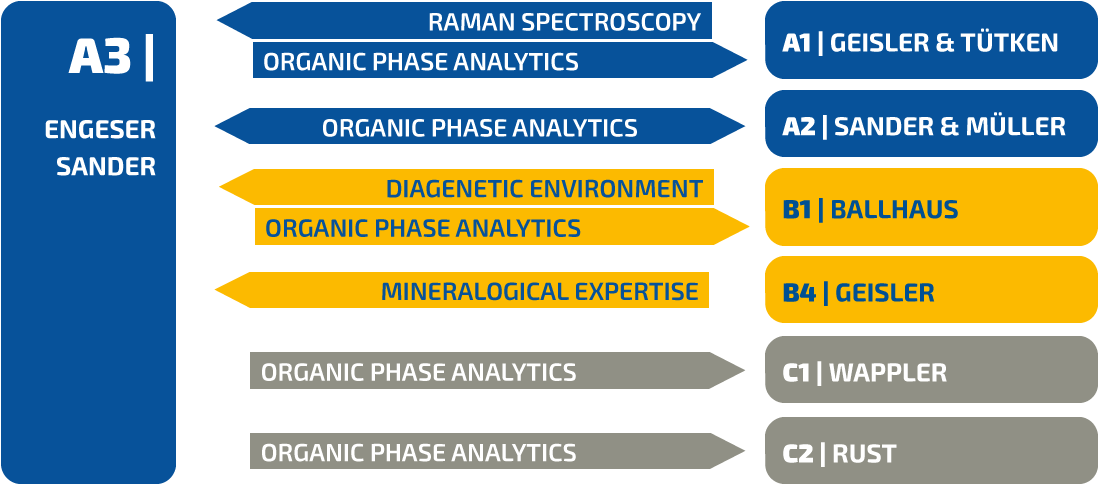
Figure 1 | Examples of different dinosaur eggs from well-defined clutches and known localities available for investigation. A, Dendroolithus egg at a Late Cretaceous excavation site in Huanglongquan in south-western Henan, China. B, Lateral view of an oviraptorid clutch in the Late Cretaceous Nanxiong Basin of northern Guangdong, China. C, Field bottom view of an oviraptorid clutch from the Late Cretaceous of Jiangxi, China.
Based on the extensive fossil eggshell collections and fruitful Chinese collaborations of the Steinmann Institute, we detected fossil egg pigments successfully and in significant amounts for the first time [1]. Key to these discoveries were the shared expertise from the fields of dinosaur reproductive biology (applicant PMS) [2,3], natural product and metabolite extraction from biological matrices (Christa Müller, project A2) and high-end analytical techniques (applicant ME; Fig. 4), e.g., liquid chromatography (HPLC) ESI mass spectrometry [4,5]. Specifically, we detected the eggshell metabolites biliverdin and protoporphyrin in eggs of the oviraptorid dinosaur Heyuanni huangi (Fig. 2). These compounds color bird eggs, and were believed to occur only in living birds.
Unpublished preliminary work documents the presence of fossil OP in these eggs, liberated by digestion in weak acid (Fig. 3).
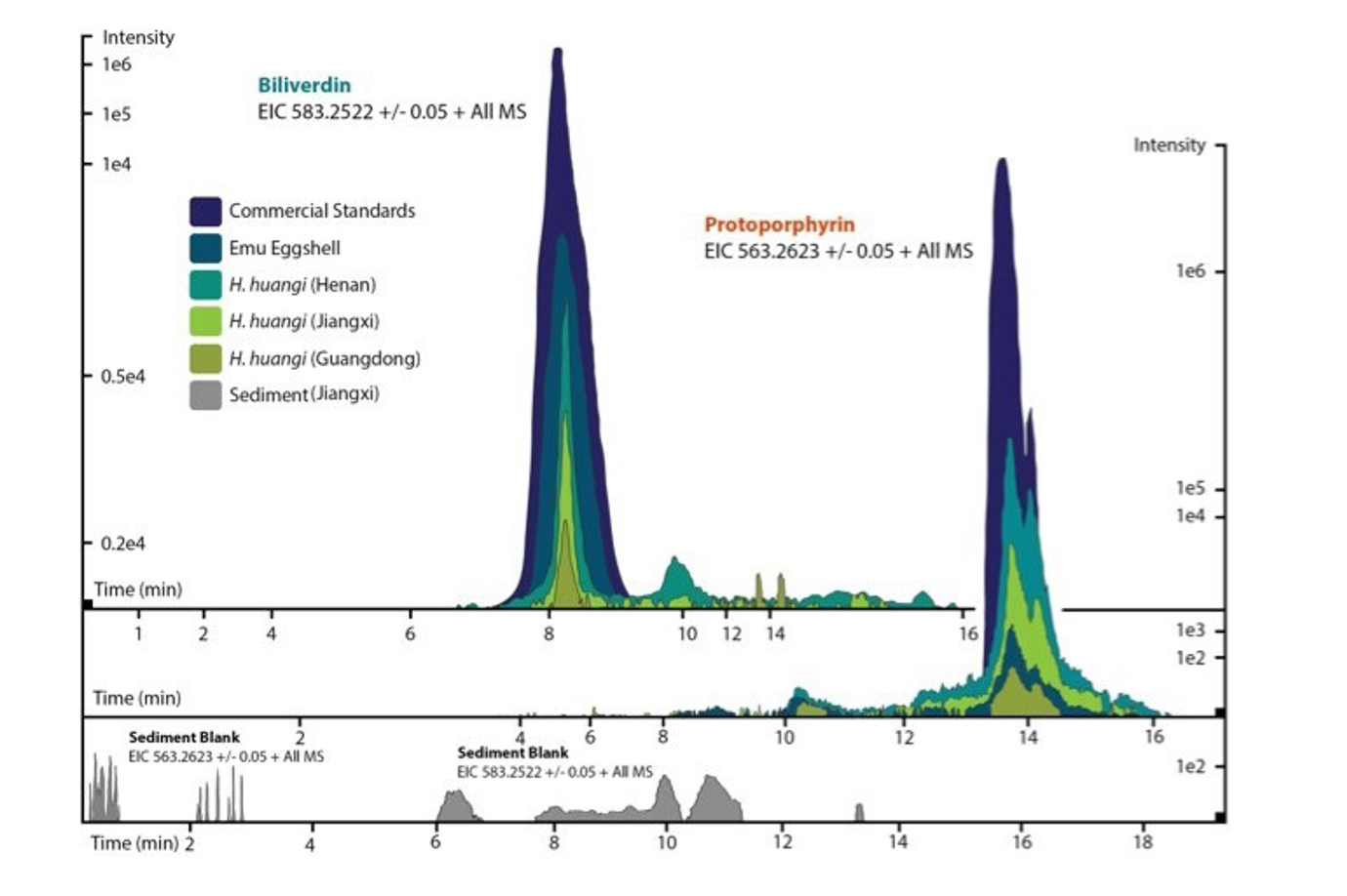
Figure 2 | ESI (+) MS extracted ion chromatgrams (EICs) for biliverdin (m / z 583.25), and protoporphyrin (m / z 563.26). EICs are depicted for the commercial standards, emu eggshell, and extracts of dinosaur eggshell (Heyuannia huangi) from different Chinese localities. From [1].
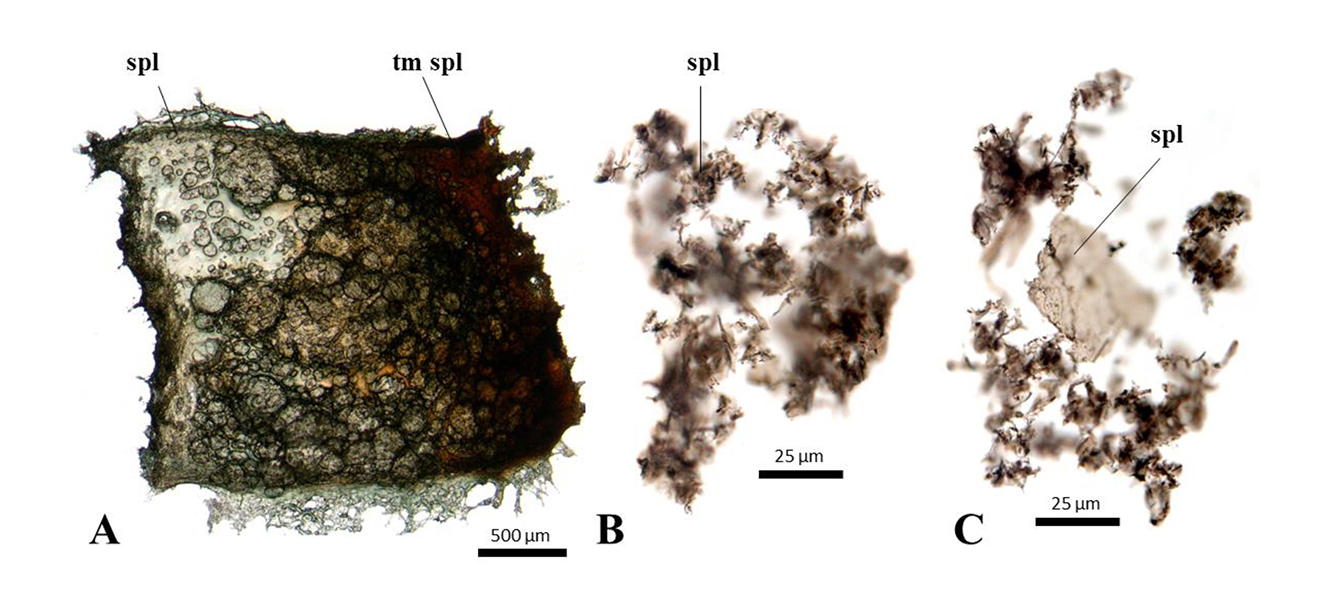
Figure 3 | Decalcified avian and non-avian dinosaur eggshell. A. Rhea (modern, in captivity, captive, Montana, USA), artificially matured spongy layer. The maturation Montana, US), artificially matured spongy layer. The maturation gradient increases to the right gradient increases to the right of the image. The original spongy layer is green of the image. The original spongy layer is green due to pigmentation, while the matured part is due to pigmentation, while the matured part is brown. B. Psammornis rothschildi brown. B. Psammornis rothschildi (Holocene, Algeria), degraded spongy layer fragments. C. (Holocene, Algeria), degraded spongy layer fragments. C. Oviraptorid dinosaur Heyuannia huangi (Late Cretaceous, Henan, China) spongy layer.
- H 1: Microscopically visible soft parts liberated from eggshell by acid digestion are altered original organic matrix.
- H 2: Organic compounds preserved in dinosaur eggshell extracts are (degraded) original compounds such as the pigments biliverdin and protoporphyrin. The eggshell provides a special environment allowing only minimal interaction with the outside world that shields the organic molecules from degradation even over very long time spans.
- H 3: Organic compounds in mineralized eggshell survive over geologic time periods in crystallite interstices.
- H 4: Organic and inorganic remains of cuticle are preserved in dinosaur eggshell.
- H 5 (contingent on H2): Pigments in eggshell inform about the evolution of bird brooding biology.
- H 6: Pigments can also be detected in invertebrate fossils showing color preservation.